Sodium Thiosulphate and Hydrochloric Acid
Sunset Yellow FCF is described as the sodium salt. The time taken for the same amount of yellow precipitate to be produced provides a method of measuring the rate of reaction at different concentrations.
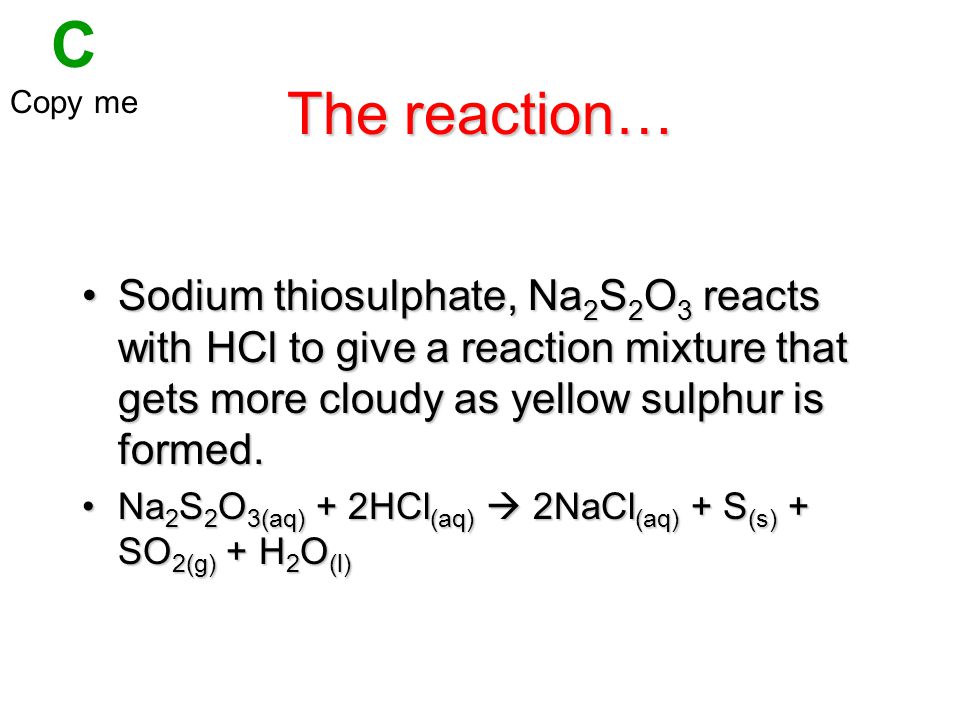
Reaction Of Sodium Thiosulphate And Hydrochloric Acid Ppt Video Online Download
Add 10 ml of hydrochloric acid and 2 g of potassium iodide stopper shake and keep in dark for 15 min.

. This involves a redox titration using sodium thiosulphate. It is a crystalline compound with five molecules of water in it. Preparation of Organic Compounds.
Kinetics Study on the Reaction between Sodium Thiosulphate and Hydrochloric Acid. How can ReAgent. Qualitative Analysis of Carbohydrates.
Sodium Thiosulphate reacts in equimolar amounts stoichiometrically with. As the sodium thiosulphate solution is run in from a burette the colour of the iodine fades. Uses for Hydrochloric Acid A highly corrosive mineral acid hydrochloric acid has many industry uses making it known as a workhorse chemical.
Chemical dechlorination of water is usually carried out by dosing of Sodium Sulphite Na2SO3 Sodium Thiosulphate Na2S203 or Sodium Bisulphite NaHSO3. Petroleum aqueous refinery condensates. Oxidation Reactions of Alcohols.
Kinetics Study on the Reaction between Sodium Thiosulphate and Hydrochloric Acid. Esterification Reaction between Alcohol and Carboxylic Acid. The first order reaction is involved between the sodium thiosulphate and hydrochloric acid.
There is a very simple but very effective way of measuring the time taken for a small fixed amount of precipitate to form. 135 - Wastes containing other reactive anions. Our curricular based PL is offered to everyone.
Na 2 S 2 O 3 aq 2HCl aq 2NaCl aq SO 2 g H 2 O l S s Here the rate of a reaction is first order with respect to sodium thiosulphate Na2S2O3aq as well as hydrochloric. The choice of which particular dechlorination chemical is to be used is site-dependeant and decisions are usually based on availability and cost reasons. In the production of different types of chlorides and metals.
6 1513 words Rates of reaction between sodium thiosulphate and hydrochloric acid Pages. H 2 SO 4. Compare the Foaming Capacity of Different Samples of Soap.
Qualitative Analysis of Carbohydrates. Qualitative Analysis of Carbohydrates. Sulfuric acid 98072 gmol.
It is because of the lower concentrations of the chemicals. Sodium Thiosulphate is a colorless odorless salt that is inorganic in nature. Oxidation Reactions of Alcohols.
4 1112 words Investigating the kinetics of reaction between sodium thiosulphate and dilute hydrochloric acid Pages. This isnt all we supply or have to offer. The diazo compound is coupled with 6-hydroxy-2-naphthalene-sulphonic acid.
Na 2 S 2 O 3 2HCl Dilute Hydrochloric Acid 2 NaCl S SO 2 H 2 O. You can find the amount of iodine liberated by titration with sodium thiosulphate solution. Esterification Reaction between Alcohol and Carboxylic Acid.
Sodium thiosulphate reacts with Hydrochloric acid reacts to form a yellow precipitate of sulphur. Sulphur dioxide SO 2 and water H 2 O are also formed but it is the solid sulphur that has the biggest impact here. 134 - Wastes containing sulphides.
Potassium chromate 40 S S Sodium thiosulphate S S Potassium cyanide saturated S S Soybean oil S S Potassium dichromate 40 S S Stannic chloride saturated S S Potassium ferri ferro cyanide S S Stannous. Sunset Yellow FCF is manufactured by diazotizing 4-aminobenzenesulphonic acid using hydrochloric acid and sodium nitrite or sulphuric acid and sodium nitrite. Calcium carbonate is a chemical compound with the formula CaCO3.
The crushed up indigestion tablet solution is titrated with hydrochloric acid to calculate how much acid the tablet can neutralise as can be seen. If you add concentrated hydrochloric acid to a solution containing hexaaquacopperII ions the six water molecules are replaced by four chloride ions. Lab report Introduction.
Reaction With Aqueous Solutions of Iodine. It is a common substance found in rocks in all parts of the world and is the main component of shells of marine organisms snails coal balls pearls and eggshells. It is a by-product of chlorine manufacture along with sodium hypochlorite and sodium hydroxide.
Hydrochloric acid 30 S S Methylsulfuric acid S S Hydrochloric acid 35 S S Milk S S Hydrocyanic acid S S Mineral oils S U. The Rate of Reaction between Hydrochloric Acid and Sodium Thiosulphate Solution Pages. Oxidation Reactions of Alcohols.
Waste brines from chlor-alkali plants. Esterification Reaction between Alcohol and Carboxylic Acid. 31 Sodium Bisulphite NaHSO3 dosing.
Preparation of Organic Compounds. In the refinement of ore. Childminders early years workers primary and secondary staff as well as lecturers technicians and those who work with young people in non-formal settings such as youth workers and in the CLD sector.
Stand the flask on a piece of paper with a cross. Hydrochloric acid is found naturally in gastric acid. Add 100 ml of water to the above mixture and titrate with sodium thiosulphate using starch as the indicator.
The biological oxygen demand BOD is a measure of water quality and can be calculated by observing how the oxygen content changes over. Kinetics Study on the Reaction between Sodium Thiosulphate and Hydrochloric Acid. Typically these solutions contain about 3-8 sodium hypochlorite and 001-005 sodium hydroxide added to reduce decomposition into sodium chlorate and sodium chloride.
Silver Nitrate AgNO3 Sodium Hydroxide NaOH Sodium Hypochlorite NaClO Sodium Thiosulphate Sulphuric Acid H2SO4 Ultrapure Water Water Purified XyleneVIEW ALL PRODUCTS. For instance the chemical name of NaCl is Sodium chloride and its common name is salt. If you add dilute hydrochloric acid to sodium thiosulphate solution you get the slow formation of a pale yellow precipitate of sulphur.
SSERC offers a vast portfolio of professional learning PL programmes for STEM educators in Scotland. In addition to removing stains in clothes sodium hypochlorite is also used to clean other types of stains such as mold and tea stains. Na 2 S 2 O 3 aq 2HCl aq 2NaCl aq H 2 O l Ss SO 2 g.
Sodium thiosulphate What is the chemical formula of Plaster of Paris. The dye is isolated as the sodium salt and dried. It is an industrial compound which has long been used in a wide range of medical applications.
ReAgent is able to formulate an endless number of organic chemical products and are on-hand to help manufacture any bespoke blend your business requires. Dewatered solids from brine treatment. Sodium Thiosulphate And Hydrochloric Acid 2.
Sodium thiosulphate sodium thiosulfate is an inorganic chemical compound described with the formula Na 2 S 2 O 3It is mostly found in the pentahydrate form hence referred with the chemical formula Na 2 S 2 O 35H 2 O. Hydrochloric Acid HCL Isopropyl Alcohol Methylated Spirits Methylene Chloride. Compare the Foaming Capacity of Different Samples of Soap.
Dissolve 0125 g of accurately weighed potassium dichromate in 25 ml of water present in a 250 ml erlenmeyer flask. Compare the Foaming Capacity of Different Samples of Soap. When sodium thiosulphate is added to a solution of hydrochloric acid an insoluble precipitate of sulphur S is formed.
Nitric Acid 69 General Use Select options. Preparation of Organic Compounds. Sodium Thiosulphate01N Hydrochloric Acid Ammonium Chloride Starch indicator ii IS 15302 Atomic Absorption Spectrophotometer and Associated equipment Burner Readout mechanism lamp for Barium Pressure Reducing valves and vents Nitrous oxide burner head T-junction valve or other switching valve.

Rate Of Reaction Of Sodium Thiosulfate And Hydrochloric Acid Youtube

How To Balance Na2s2o3 Hcl Nacl H2o S So2 Youtube

Sodium Thiosulfate Hydrochloric Acid Experiment Video Lesson Transcript Study Com

Experimental Chemistry Why Would Rinsing The Flask With Acid In This Experiment Affect Its Accuracy Chemistry Stack Exchange
No comments for "Sodium Thiosulphate and Hydrochloric Acid"
Post a Comment